Corrosion resistance mechanism of ZAM
Mechanism of corrosion resistance on flat section
Al and Mg in the coating layer of ZAM® combine to form a fine, tightly adhered zinc-based protective film on its coating surface as time passes.
This protective film suppresses corrosion of the ZAM® coating.

Galvanized coating layer also forms a protective film on the surface. This protective film, however, is not as fine as in ZAM®, and less adhesive (see photo at right).
In contrast, the protective film formed on the coating surface of ZAM® is excellent in both f inenes s and adhes ion, and consequently it inhibits permeation of cor rosion factors, preserving high corrosion resistance over a long period.

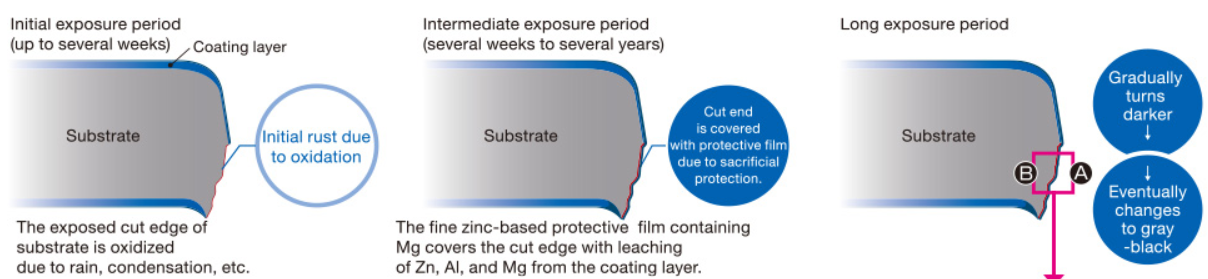
Mechanism of corrosion resistance on cut edge
Excellent corrosion resistance is achieved on cut edge parts by covering the ends with a fine zinc-based protective film that contains Al and Mg leaching from the coating layer.
 (Thickness: 3.2 mm, coating weight: 150/150 g/m2, post-treatment: chromate 50 mg/m2)
(Thickness: 3.2 mm, coating weight: 150/150 g/m2, post-treatment: chromate 50 mg/m2)
Note: The color and the speed of change in color depend on sheet thicknesses and exposure environments (region, installation location, aspect, etc.).
Cross-sectional structure and distribution of elements formed on cut edges
after 18 months of outdoor exposure test
 (Thickness: 2.3 mm, coating weight: 130/130 g/m2, post-treatment: chromate 50 mg/m2)
(Thickness: 2.3 mm, coating weight: 130/130 g/m2, post-treatment: chromate 50 mg/m2)
Source: Nippon Steel



